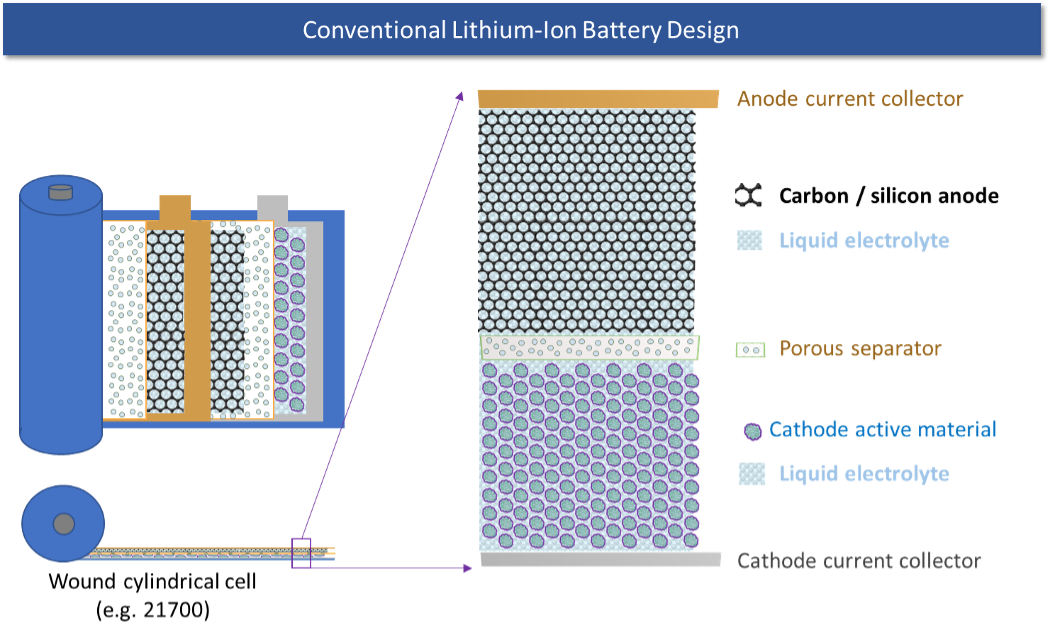
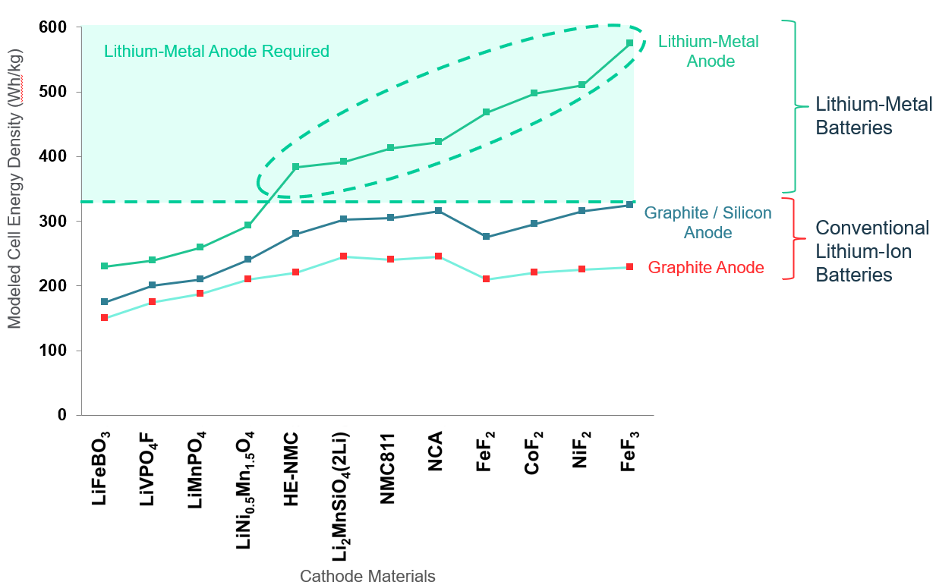
The potential increase in energy density for a lithium-metal anode battery has been known since the mid-1970s. However, it has also been known that lithium-metal anodes do not work with conventional liquid electrolytes due to the twin issues of dendrite formation when a battery is being charged and rapid impedance growth from a chemical side-reaction between the liquid electrolyte and the lithium metal. Dendrites are needle-like formations of lithium metal which can grow across the separator and short-circuit the cell. Impedance refers to the internal resistance of the cell; growth in this resistance reduces the energy capacity of the cell as well as its ability to work at high rates of power.
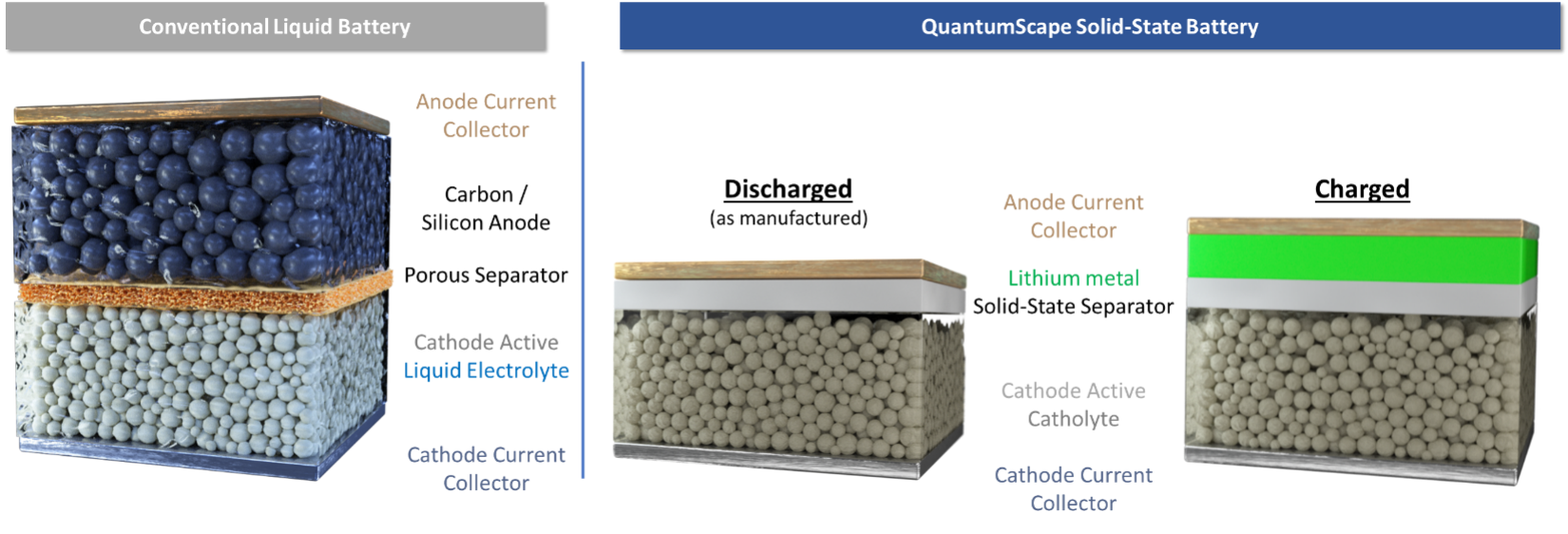
Thus, it is widely believed that to make a lithium-metal anode battery, one needs a solid-state separator which is roughly as conductive as a liquid but resists dendrite formation and does not react with metallic lithium. For 40+ years, the industry has been searching for such a material.
The Promise of the Solid-State Lithium-Metal Battery
It turns out that the lithium-metal anode enabled by such a solid-state separator could address not only the energy density problem, but also a number of the other limitations of conventional lithium-ion batteries, since many of these stem from the carbon anode as well, including:
- Energy density: Since the carbon that makes up the anode takes up space and has mass, eliminating it increases the energy density of the cell.
- Power density/fast charge: The lithium that cycles through the cell into the anode has to diffuse into the carbon at a rate that is governed by fundamental material properties of graphite. Any attempts to drive the lithium ions into the carbon anode particles faster than this natural rate of diffusion can result in the lithium “plating” on the surface of the particle instead of diffusing into it causing capacity loss and failures. Eliminating the carbon removes this limitation, allowing for fast charge without any adverse impacts.
- Cycle life: The cycle life of the cell is partly limited by an irreversible chemical side reaction (i.e., an unwanted reaction) at the interface of the carbon particle and the liquid electrolyte, which consumes a little bit of lithium on every charge-discharge cycle, resulting in a cumulative loss of capacity (and therefore energy) over the life of the cell. With no carbon in the anode this side reaction should be eliminated, resulting in improved cycle life for the cell.
- Safety: The polymer separator and the liquid electrolyte used in lithium-ion batteries are both hydrocarbons and are combustible. Starting a fire requires three elements: A fuel, an oxygen source, and a heat source. Because the electrolyte — a fuel — is in direct contact with the cathode, which is an oxide, the only other element needed to cause a fire is a heat source. Many abuse conditions, from internal short-circuits to accidents, can provide that heat source. Replacing the polymer separator with a solid-state ceramic separator that is thermally stable to very high temperatures and does not burn (since it is already oxidized) both reduces the fuel content of the cell and provides a thermally stable barrier between the anode and cathode.
- Cost: The costs of the materials associated with the carbon anode and the anode electrode manufacturing process can be eliminated by replacing the carbon anode with a lithium-metal anode. In addition, the conventional formation process, one of the most expensive parts of the battery manufacturing process in which the assembled cells must sit in storage for a period of weeks to form the proper interfaces on the electrode particles and allow for the identification of manufacturing defects, can be dramatically simplified.
The Challenge
This is the promise of solid-state lithium-metal batteries and is why the industry is so excited about the possibilities. However, making the solid-state ceramic separator required to deliver on this promise has turned out to be a very difficult challenge. More specifically, such a separator needs to:
(a) have lithium-ion conductivity similar to or better than today’s liquid electrolytes;
(b) be chemically and electrochemically stable to lithium metal; and
(c) resist the formation of lithium-metal dendrites.
Despite decades of work, the industry has not found any separator materials capable of meeting these requirements.
Comparing Separator Materials
Many classes of separator materials have been tried, none of which have been shown to simultaneously meet the key requirements. These include:
- Polymers: Lithium-conducting polymers, such as polyethylene oxide, were initially thought to be a candidate for a solid-state separator. Unfortunately, they are generally deficient on all three of the requirements outlined above. First, their conductivity is too low, requiring elevated temperatures to operate. Second, the poor stability of polymers in contact with lithium metal results in impedance growth over life and requires the anode to use a lithium foil to supply excess lithium to the cell, reducing energy density and increasing cost. Third, they are too soft to prevent penetration of lithium-metal dendrites through them. In addition, they are not stable above 3.8 volts, further compromising energy density by requiring a low energy cathode material.
- Sulfides: The discovery of lithium-conducting sulfides, such as LGPS by Professor Ryoji Kanno in Japan in 2011, generated excitement because they are highly conductive of lithium ions, addressing requirement (a) above. However, they are among the most thermodynamically unstable of the widely investigated solid-state electrolytes – reacting on both the high voltage cathode side and the low voltage lithium side. To offset this reactivity, the cathode materials are typically coated. Unfortunately, these coatings often raise the internal resistance of the cell and make the cell unable to perform at high rates of power or low temperatures. But most fatally, it turns out that despite years of work, sulfides have not been shown to prevent dendrites at low temperature and high charge rates, making them simply unusable in commercial battery systems for EVs.
- Oxides: Lithium conducting oxide separators were discovered over the past few decades, but while some oxides have sufficient conductivity and stability towards lithium metal, conventional oxides, too, have been unsuccessful at suppressing dendrite formation at automotive charge rate requirements.
- Composites: Some groups have worked on composites consisting of polymers and ceramics, in hopes that they could capture the “best of both worlds” – the ease of working with polymers with the hardness of ceramics. Unfortunately, such approaches end up instead with the “worst of both worlds”, resulting in an unstable material that fails to prevent dendrites, as dendrites appear to grow through the interface between the two materials.
- Liquids: Some groups continued to work on conventional liquid electrolytes, but these efforts are still challenged by the twin problems of dendrite formation and impedance growth from the chemical side-reaction between the liquid and the lithium metal.
It is important to note that if one has a system using any of the foregoing separator materials, it is still possible to make cells and report cycling results, but this cycling has to be done under compromised test conditions. In particular, the following are some of the most used compromises:
- A carbon or carbon-silicon anode instead of lithium metal: Reverting to a hosted anode sacrifices the benefits of lithium-metal anodes, such as energy density, fast charge, cycle life, safety, and lower cost. Thus, these approaches are not the step-change in performance required for mass market EVs.
- Low current density: At low current density, such as 1-2 mA/cm2, even liquid can be made to cycle with lithium metal. However, such low current densities are not useful for automotive applications.
- Elevated temperatures or pressures: At elevated temperatures, lithium metal is softer and less likely to form dendrites. In addition, high temperatures increase the conductivity of materials like polymers and sulfides and reduce the resistivity of cathode coatings. However, requiring elevated temperature makes the cell impractical and too expensive for most commercial applications. Elevated pressures similarly provide a way to “squeeze” lithium into smoother structure, but overly high pressures such as those above 10 atmospheres are simply impractical even in automotive applications.
- Low cycle life: Because of the stochastic nature of dendrites and the progressive nature of impedance growth, many cells made with materials that do not meet the above requirements can deliver some cycles, but not enough to be commercially viable, and the cells are not reliable enough to be usable in real applications.
- Excess lithium on the anode: Some efforts start with an excess layer of lithium on the anode, which makes the process of plating lithium easier, but at the expense of energy density and cost, rendering these approaches impractical for automotive applications as well.
Key Questions
Recently, there have been a number of announcements and claims relating to solid-state batteries. The first question to ask when evaluating solid-state cell claims, is whether the cells use a lithium-metal anode or a conventional hosted (carbon or carbon-silicon) anode. If they do use a hosted anode, the key performance metrics for these batteries will be similar to conventional lithium-ion batteries and not realize the benefits of the solid-state lithium-metal approach (significantly higher energy density, fast charge, life, and cost). A number of recent claims fall into this category.
If the solid-state cell in question does use a lithium-metal anode, the next question to ask is whether it can perform under uncompromised test conditions, including near and below room temperature and high current density (i.e., high rates of power such as 1-hour charge or 15-minute charge). Specifically, what cycle life does the cell deliver at near-room temperature (~ 30°C) with automotive rates of power (>3 mA/cm2, required for one hour charge)? If the cells cannot perform under these conditions, we believe they are not commercially viable. Many of the other solid-state lithium-metal announcements fall into this category.
QuantumScape’s Approach
Many solid-state announcements either do not show any data at all, or leave out some of the above parameters when they report data, leaving an incomplete picture at best. At QuantumScape, we have developed a solid-state ceramic separator capable of meeting these requirements without requiring the compromised test conditions described above. We have presented data showing single-layer versions of our solid-state lithium-metal cells can cycle more than 1000 cycles and retain over 90% of their initial energy when cycling at aggressive 1C rates of power, near room temperature, and with modest pressure. More recently, we have presented data showing multilayer cells cycling to close to 800 cycles with similar capacity retention.
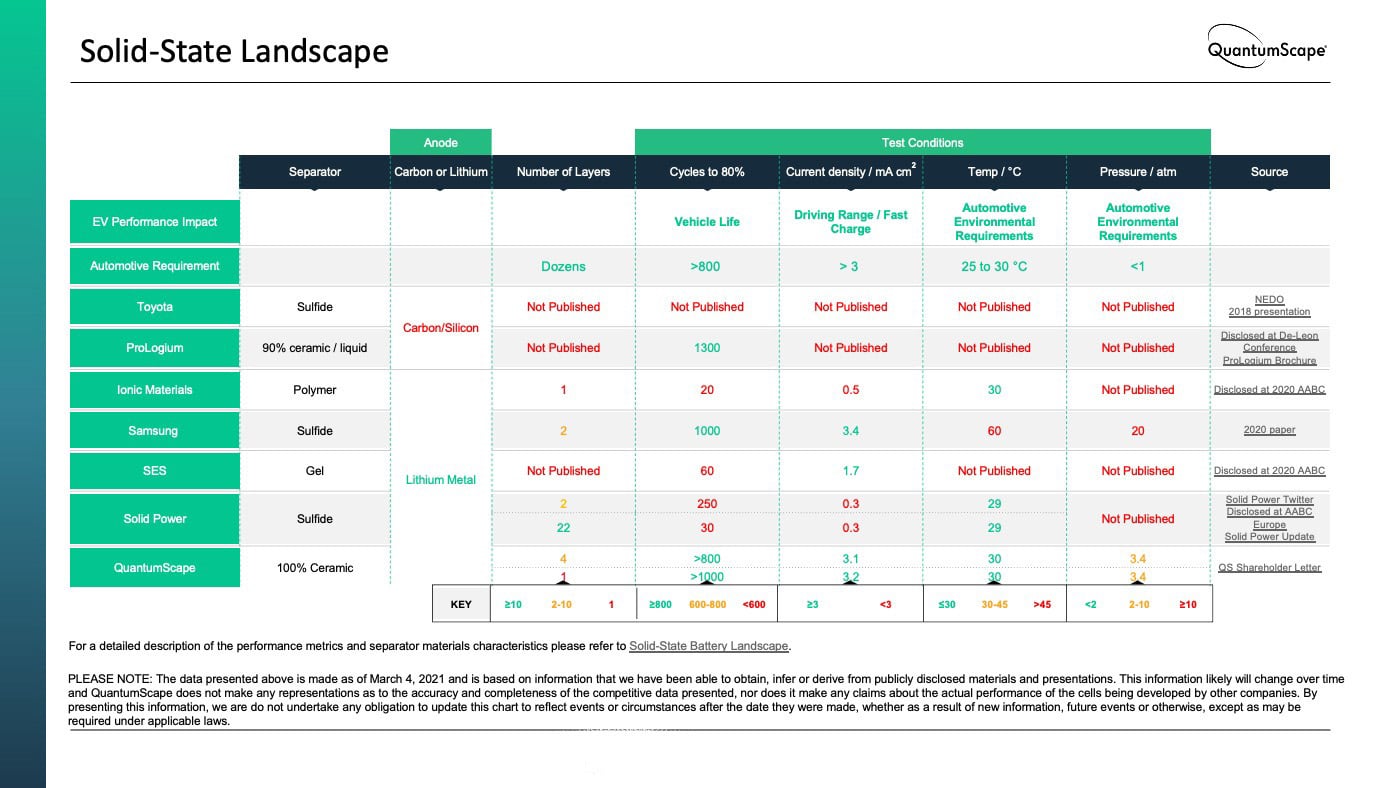
We have compiled data on key performance metrics across major solid-state battery technology efforts based on information we have been able to obtain, infer or derive from publicly disclosed materials and presentations. This data is presented in the chart below, and is current as of March 4, 2021.
We hope that this paper helps our stakeholders understand the broader technology landscape of solid-state battery technology and QuantumScape’s distinctive approach.