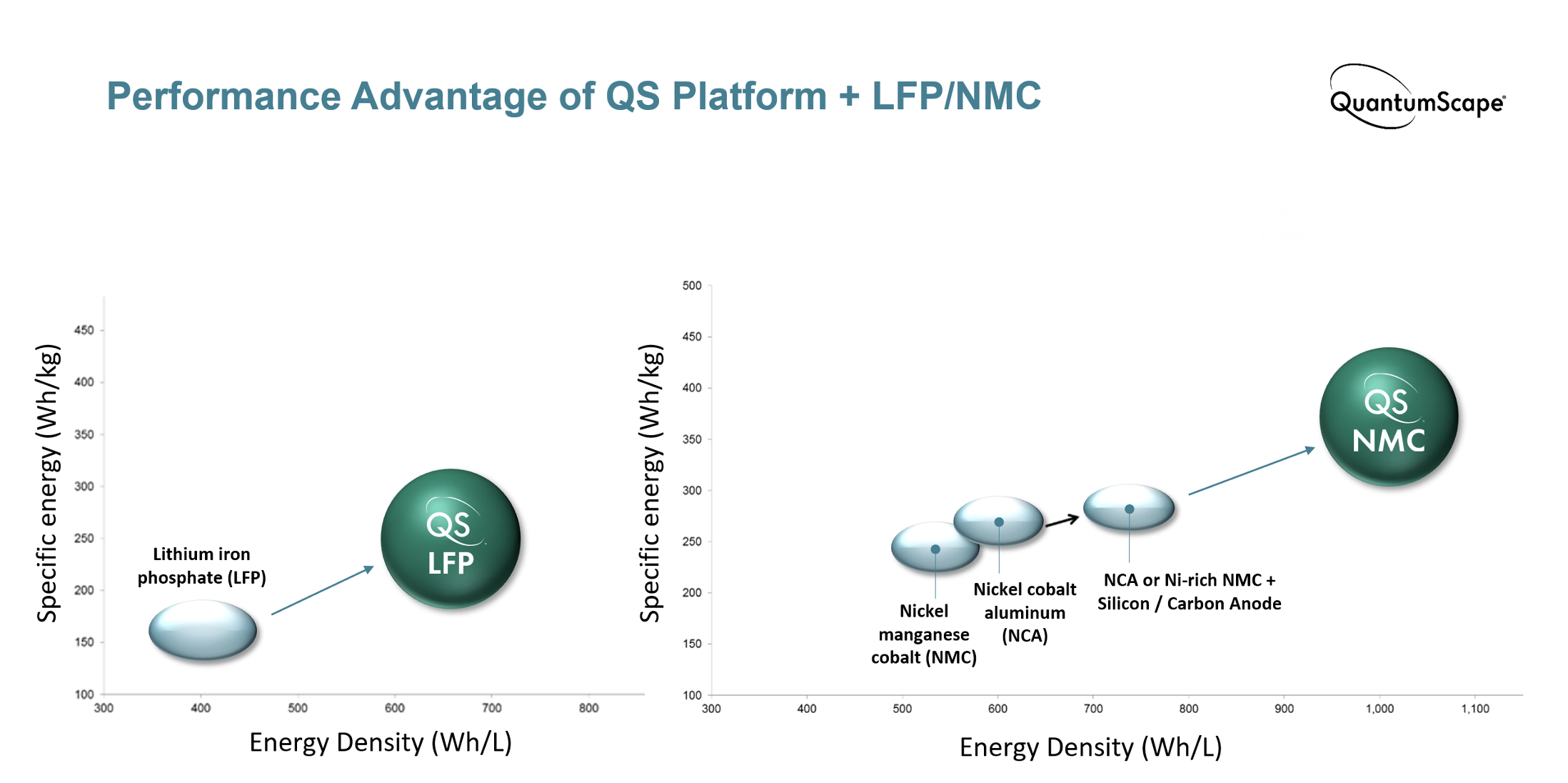
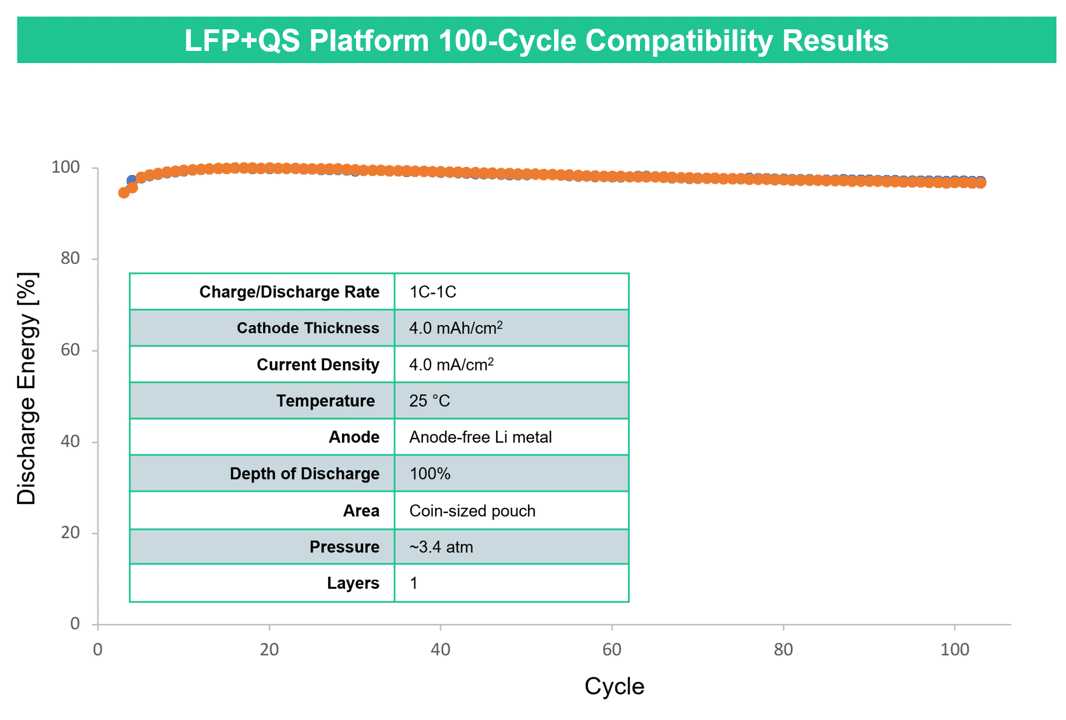
LFP: Challenges and Opportunities
Like many inventions that have made the lithium-ion battery possible, LFP cathode material was discovered in the lab of Nobel-laureate Professor John Goodenough.[1] Unlike other common oxide cathode materials, LFP is a polyanion compound; that is, it’s composed of more than one negatively charged element (oxygen and phosphorus). Its atoms are arranged in a crystalline structure called olivine, which forms a 3D network for intercalation of lithium ions; this is in contrast to the 2D slabs that form layered oxides like NMC and NCA.
The main challenge of working with LFP cathode material was the poor transport of electrons and lithium ions through the polyanionic olivine structure. This poor transport contributed to high cell resistance and limited power performance. However, reducing the size of LFP particles to nanoscale and the addition of carbon coatings around the particles have significantly improved LFP’s power performance, to the point that it’s now often used in power cells.[2]
LFP has several advantages over other popular cathode chemistries:
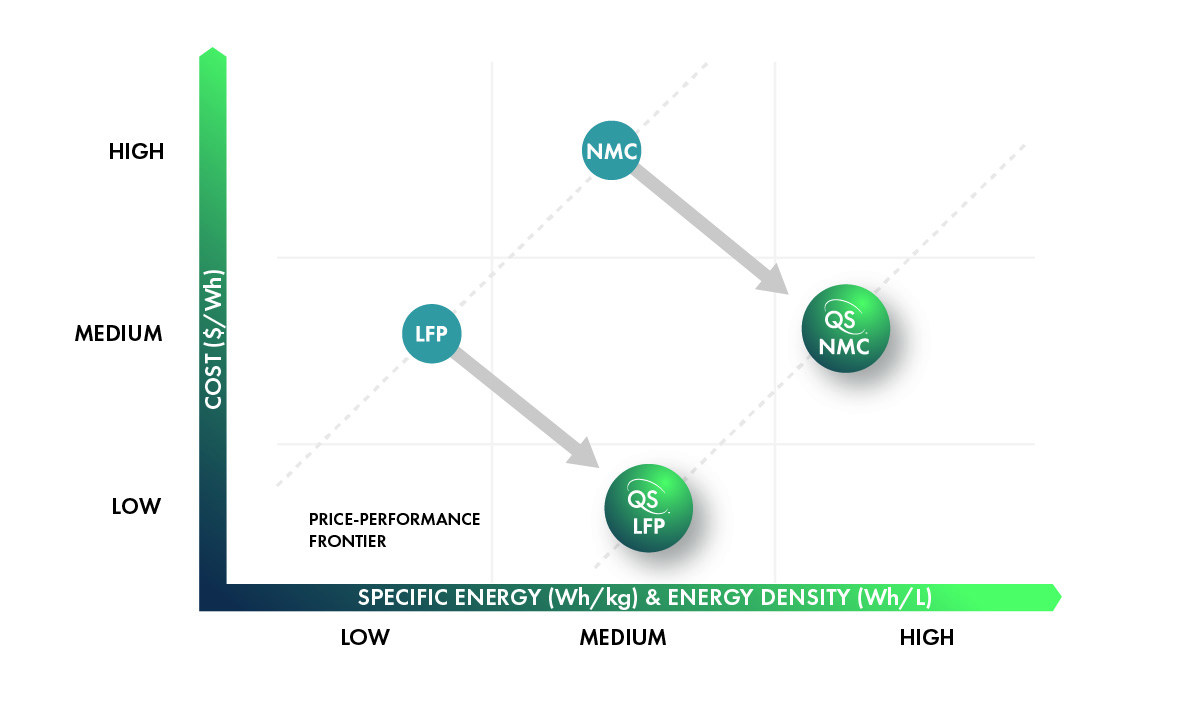
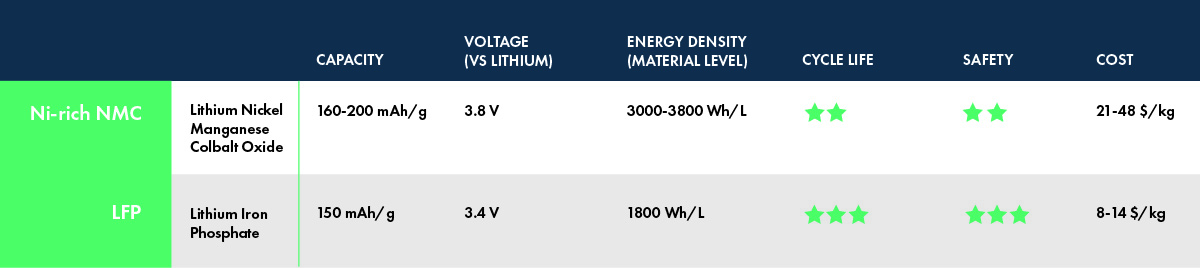
LFP is widely known for its low cost, with some estimates putting it as much as 70% lower per kilogram than nickel-rich NMC. The cost advantage comes from its chemical composition – iron and phosphorus, two earth-abundant elements that are mined at enormous scales across the globe and are widely used in many industries. In contrast, NMC cathodes contain nickel and cobalt, which are scarcer and, in the case of cobalt, mined under controversial conditions. And although nickel-rich NMC replaces most of the cobalt with nickel, nickel is still orders of magnitude less abundant than iron (0.0084% vs 5.63% of the earth’s crust[3]) and its global production volume is hundreds of times less than iron.
LFP is very thermally stable, which means it takes higher temperatures to decompose and burn.[4] In the event of thermal runaway, LFP releases one-sixth of the heat of nickel-rich NMC. This means that cooling and safety systems in the battery pack can be simplified without sacrificing safety, thus providing additional cost savings and decreasing the risk of expensive battery recalls in the case of manufacturing defects.
With this enhanced safety, battery packs can be constructed with more densely packed cells (e.g., cell-to-pack architecture), increasing the effective energy density. In commercial EV battery packs based on NMC and NCA, cells account for 40% or less of total pack volume, whereas the most advanced LFP cells can achieve 60% pack volume utilization.
LFP often offers better cycle life compared to NMC.[5] One reason for this is its lower operating voltage; given the high-voltage instability of common liquid electrolytes, LFP cells are less prone to side reactions that reduce capacity and increase cell resistance. Another reason is that LFP cathodes are more structurally and chemically stable than NMC cathodes, so they can withstand more cycles without breaking apart and losing their ability to store lithium ions. LFP batteries could conceivably power a car for over a million miles.
All of these characteristics make LFP one of the more cost-effective solutions available for EV batteries, and its market share has grown substantially over the past few years.