C-rate also is a relative metric. Many important aspects of battery behavior (such as resistance to dendrites) depend on the absolute current density, the amount of electrical current that goes through the battery divided by the area of its layers. For a given C-rate, the current density will be a function of the loading of the cathode, which is closely related to its thickness. Thus, when comparing C-rates, it’s important to ensure the cathode loading is at commercially relevant levels: for EVs, this is typically in the range of 2.5-5 mAh/cm2.
What do EV drivers want?
To be a viable alternative, EVs should perform at least as well as their internal-combustion engine (ICE) counterparts: deliver high levels of power during acceleration, operate in all driving conditions, and require minimal time to recharge.
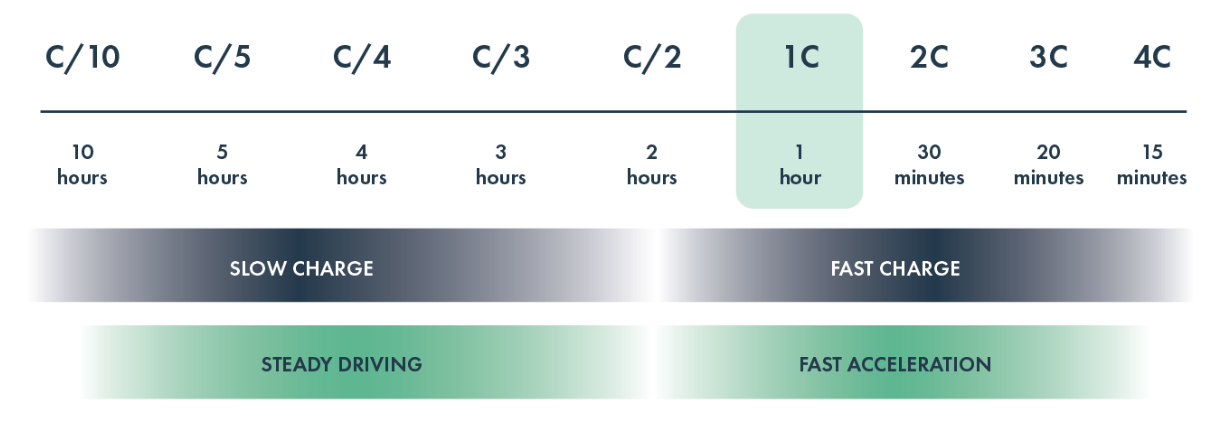
In an ideal world, a battery would perform at extremely high C-rates all the time. However, the higher the C-rate, the more difficult it is for a battery to deliver reliable performance. Higher C-rates increase the rate of degradation in the battery, reducing range and shortening the vehicle’s lifespan. Faster charging can also cause dangerous dendrites to form. These hurt the battery’s lifespan, can lead to cell failure, and in some extreme cases have been known to cause fires. Consequently, EV battery chargers do not sustain charging rates higher than 2C for longer than a few minutes before the charging rate is reduced to avoid causing damage to the battery.
This means that today’s best EV batteries can still only be charged relatively slowly compared to the few minutes it takes to fill a combustion engine gas tank. Of course, EVs have the advantage that they can be recharged at home and overnight, and so fast-charging is usually more desirable when taking trips longer than a single charge (typically around 300 miles) would allow. And the good news is that most batteries regularly charged at slow C-rates will retain their original range for longer than similar batteries repeatedly charged at fast rates.
However, we believe that mass-market EV adoption will require a more comparable experience ICEs. To achieve this, battery technology must be improved to enable a car to charge at substantially faster rates so that drivers can “fill” their batteries in minutes instead of hours. This is a key reason why certain next-generation battery technology is so important.
Not all battery technology is equal
If current battery technology risks dendrite formation if charged too quickly, are next-generation battery technologies going to solve this problem? Unfortunately, it has been found that not every new battery technology is capable of delivering improvements to fast-charging performance. For example, in our blog post on sulfides, we explained why we believe that many of the newly announced solid-state lithium-metal batteries based on sulfide electrolytes are unlikely to deliver improvements in charging performance over conventional lithium-ion batteries. Similarly, due to the twin issues of dendrite formation and resistance growth, we believe most liquid electrolyte-based lithium-metal batteries are also unlikely to be able to deliver fast charge performance. Though they appear to successfully charge at relatively slow rates, such as C/5, we believe these fundamental limitations to many of the sulfide- and liquid-based technologies are likely to put super-fast charging speeds out of reach.
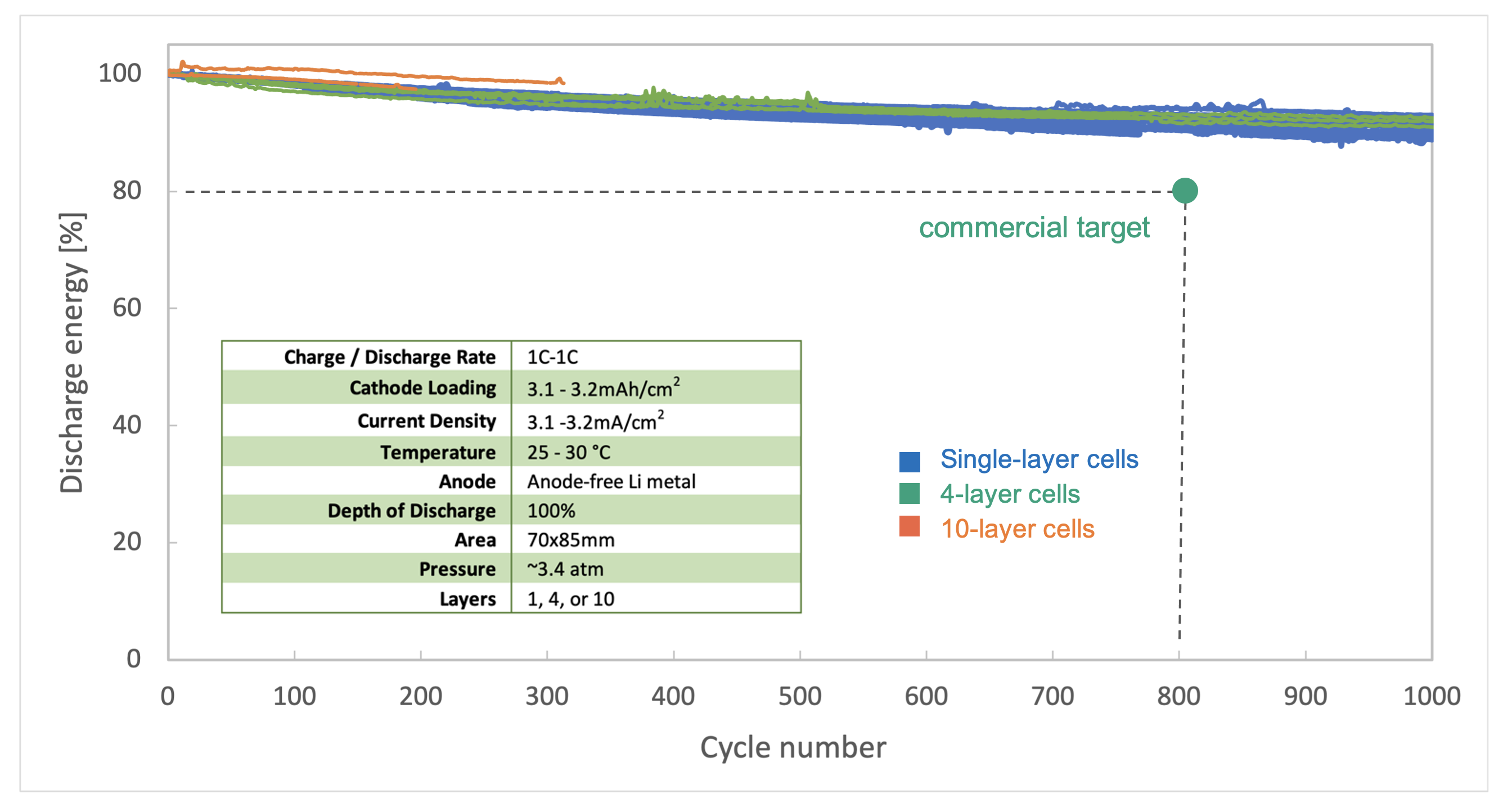
In contrast, our ceramic solid-electrolyte separator enables the use of a lithium-metal anode in its fully charged state. This lithium-metal anode replaces the graphite anode, one of the key bottlenecks to fast charging in current EV batteries. The test results of our batteries using our solid-state lithium-metal anodes show better than 80% energy retention after 800 charging cycles with repeated 1C rates of charge and discharge, the equivalent of over 240,000 miles for a car with a 300-mile range. In short, charging our lithium-metal batteries at a relatively high 1C rate does not cause a dramatic drop in range over the battery’s lifespan.
We believe the data we have shown demonstrates that our fundamental technology, when scaled to a size that is useful for EVs, can deliver the fast-charging speeds that make EVs competitive with ICE vehicles. Our ultimate goal is to make a battery for EVs that can recharge from a low state of charge to 80% in 15 minutes, empowering drivers to switch to zero-emissions cars without having to tolerate the inconvenience of slower recharging currently associated with today’s EVs. We recognize that to make an impact in the real world, we need batteries that don’t compromise on power, charge time, or battery life, and that’s precisely why we design and test our batteries using C-rates that are more reflective of real-world conditions.