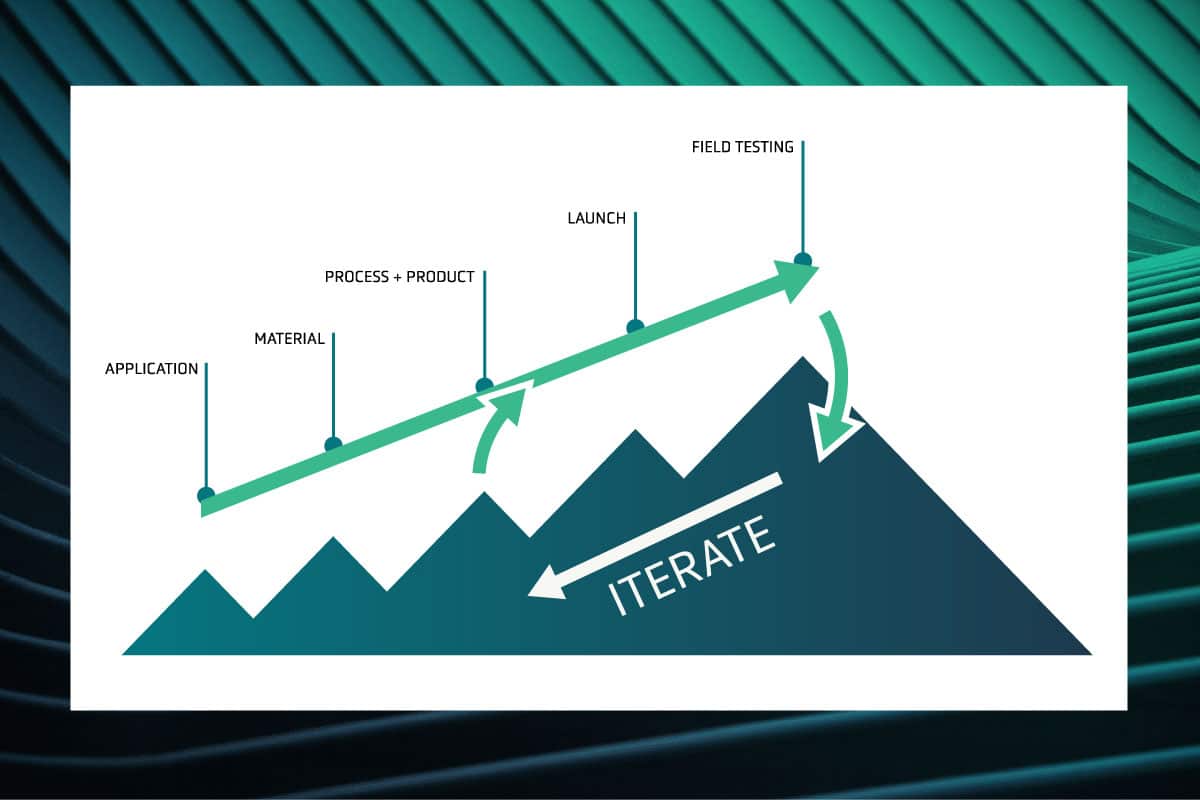
In our blog post on sulfides, we outlined some of the limitations we believe make sulfide-based solid electrolyte separators unsuitable for enabling lithium-metal anode technologies. Here we’ll review the challenges facing two other approaches: liquid electrolytes[1] and solid polymer electrolytes.[2] We group them together because both liquid and solid polymer electrolytes typically consist of organic materials, which give them similar properties.
Lithium metal’s fundamental properties make it the ideal anode material for a lithium-based battery system,[3] as it offers superior energy density (and therefore vehicle range) and can potentially solve the fast-charge bottleneck from which legacy lithium-ion batteries suffer. These benefits explain why battery scientists have been researching the potential for using lithium-metal anodes with liquid and polymer electrolytes since the 1970s.[4] More recently, some polymer-based approaches have even seen limited commercial use. Liquid and polymer electrolytes are superficially attractive technology pathways since they are commonly used in legacy lithium-ion batteries with graphite anodes.
Despite the long history of unsuccessful attempts, new companies continually take up the effort to work with liquids or solid polymers with a lithium-metal anode. But test data we’ve seen[5] shows they still suffer from the same underlying problems — slow rates of charge, poor safety and reliability, and high cost — which is why we believe these efforts will not be commercially viable in vehicles.
The most serious problems that both liquids and solid polymer electrolytes face when paired with high-performance lithium-metal anodes are:
- Dendrites and safety
- Low charge rates
- Expensive lithium foil
Dendrites and safety
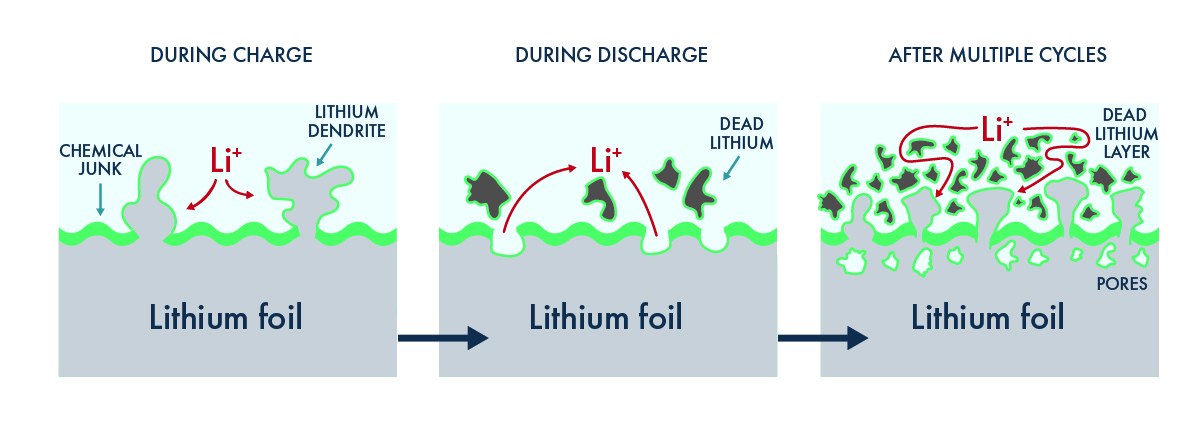
Dendrites are root-like structures of pure lithium that can grow from the anode of a lithium-metal battery cell. In our sulfides article, we describe the dendrite problem in detail. Based on our extensive experience and research on dendrite formation and prevention, we think that neither liquids nor solid polymers can prevent dendrites under the kinds of conditions, such as fast charging rates, required in automotive applications. The reason is simple: the polymer separators in liquid, gel and solid polymer batteries simply aren’t strong enough to resist the growth of these structures, and when the dendrites inevitably reach the cathode, they kill the battery.
And it doesn’t stop there. Dendrites create a short circuit within the cell that can also spark a fire. This is a problem because both liquid and polymer electrolytes are flammable, energy-rich materials, which means a potential fire has plenty of fuel to burn. It’s important to note that safety tests carried out in newly fabricated cells likely won’t show the dramatic impacts on safety that manifest only after the battery has been cycled repeatedly. This is because, as dendrites form, the surface area of the lithium-metal anode increases and makes the cell more susceptible to catastrophic thermal runaway.[6] Not only do such fires pose a risk to driver safety, but EV recalls can cost billions of dollars, potentially making solid polymer and liquid-based systems major liabilities.
Liquids and solid polymer electrolytes haven’t been shown to prevent the destructive effects of dendrites, and this is the main motivator behind the search for a solid electrolyte material that can prevent them. But dendrites are not the only dealbreaker these technologies face.
Low charge rates
Liquid and solid polymer electrolytes are inherently unstable and corrode lithium metal,[7] forming chemical junk[8] that increases the internal resistance of the cell. This problem is compounded by dendrite formation: as new dendrites grow, fresh lithium is exposed, creating more junk. Moreover, lithium dendrites can separate from the anode surface, forming clumps of dead material that will further impede the movement of lithium ions. As inactive debris accumulates, it causes a fade in the average voltage of the cell and reduces the energy that the battery can deliver to the vehicle.[9] Importantly, reporting capacity retention of cells with lithium-foil anodes can hide this problem, as the excess of lithium in the foil compensates for the capacity losses, but energy tests of the same cell will show precipitous decline when the cell is cycled. Since energy is what makes a car go, the increased resistance renders such cells unsuitable for use in automotive applications.